On June 4, 2023, Escugen's ESG401 (Trop2 ADC) made a notable appearance at the 2023 ASCO meeting, presenting preliminary data from its first-in-human study in the form of a poster.
Study Title:
Preliminary results from a First-in-human Study of ESG401, a Trophoblast Cell-Surface Antigen 2 (TROP2) Antibody Drug Conjugate (ADC), in Patients with Locally Advanced/Metastatic Solid Tumors.
Study Methods:
This is an open-label, dose-escalation, and cohort expansion Phase I/II study. Eligible patients are males and females aged 18-75 years, with an ECOG status of 0-1, histologically or cytologically confirmed advanced or metastatic solid tumors, and no effective standard treatment or intolerance to standard treatment. Patients with CNS metastases that are stable or improved after complete resection and/or radiotherapy are also eligible. Eligible patients receive intravenous infusions of ESG401 according to the specified dosing schedule (every 21 or 28 days as one cycle) until disease progression, intolerable toxicity, withdrawal of consent, death, or other reasons specified in the protocol.
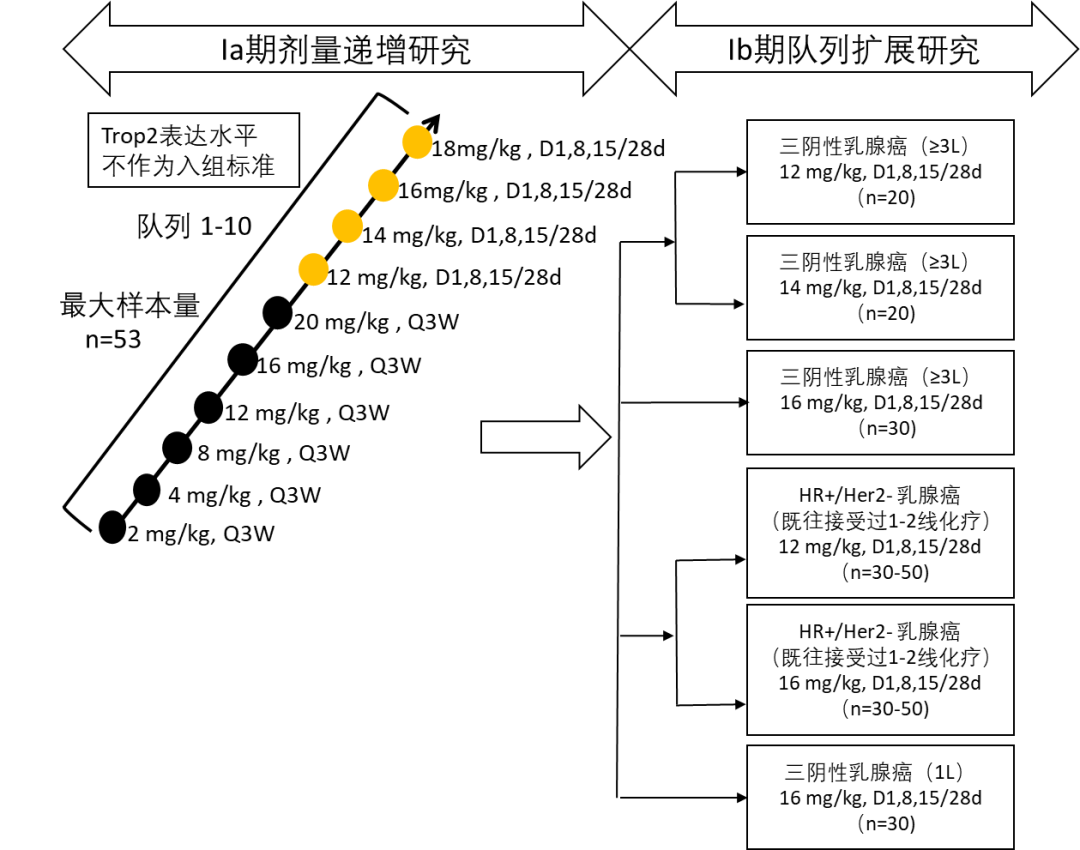
Primary endpoints are the safety and tolerability of ESG401, and determination of the MTD and RP2D. Secondary endpoints include pharmacokinetics, immunogenicity, and preliminary efficacy evaluations (ORR, DCR, DOR, TTR, PFS, and OS as determined by the investigator). Registration information: NCT04892342.
Study Results:
•Baseline Characteristics:
A total of 35 patients were enrolled, including 1 patient with endometrial cancer, 16 with triple-negative breast cancer (TNBC), 16 with HR+/HER2- breast cancer, and 2 with HR-/HER2+ breast cancer. The median age of the enrolled patients was 53 years (range: 32-70); the median number of previous treatment lines after metastasis was 4 (range: 1-12). At baseline, 63% of patients had liver metastases, 60% had lung metastases, and 11% had brain metastases.
•Safety:
Treatment-related adverse events (TRAEs) occurred in 94.3% (33/35) of patients, with Grade 3 or higher TRAEs in 34.3% (12/35). The most common ≥Grade 3 TRAEs (≥10%) were neutropenia and leukopenia. The incidence of SAEs related to the study drug was 5.7% (2/35). No Grade 3 or higher thrombocytopenia, diarrhea, rash, or stomatitis was observed, and no interstitial lung disease (ILD) was reported. In the 20 mg/kg Q3W dose group, one participant reported two DLT events (Grade 4 neutropenia and Grade 3 febrile neutropenia); the MTD has not yet been reached.
•Efficacy:
Among the 33 evaluable patients, 12 achieved PR, with an objective response rate (ORR) of 36.4%, and 9 achieved SD (with 4 maintaining SD for ≥24 weeks), resulting in a disease control rate (DCR) of 63.6%. Additionally, the ORR in the highest dose group was 66.7%, with a DCR of 83.3%; the ORR in TNBC patients at effective doses was 36.3%, with a DCR of 63.6%; the ORR in HR+/HER2-BC patients at effective doses was 61.5%, with a DCR of 76.9%.
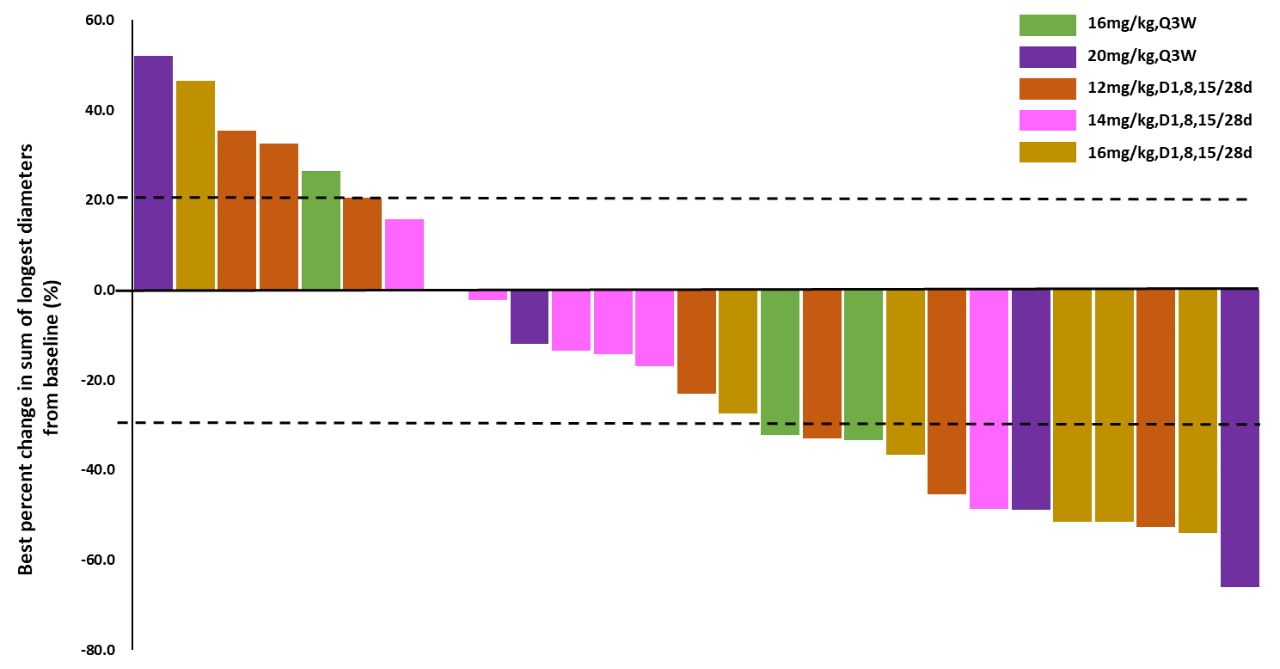
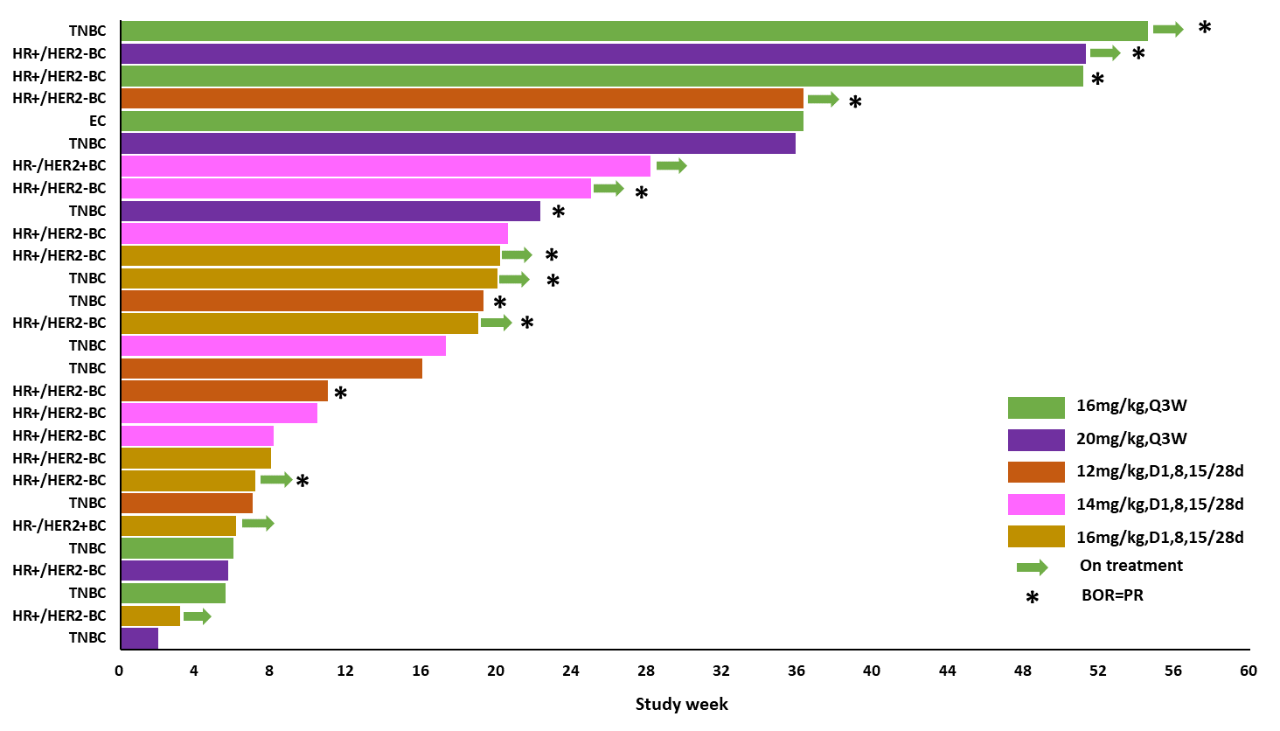
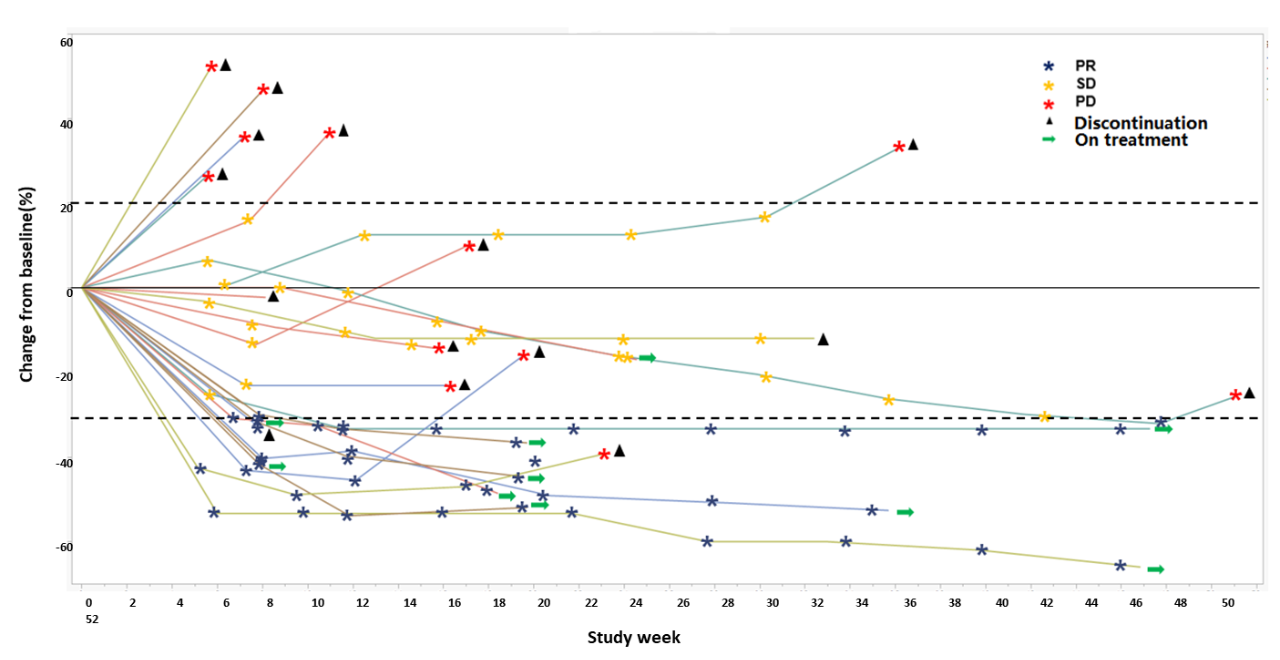
Highlights Summary:
ESG401's unique stable linker design ensures that the drug remains in a stable ADC form in serum and normal tissues, effectively reducing the risk of off-target toxicity. Preliminary study results indicate that ESG401 has good safety, an advantageous adverse reaction profile, and allows for long-term treatment of participants. The main adverse reactions of ESG401 are imperceptible and well-managed neutropenia; the incidence of Grade 3 or higher anemia is low, with no Grade 3 or higher thrombocytopenia, diarrhea, rash, or stomatitis observed; no interstitial pneumonia has been reported. ESG401 has a broad therapeutic window and two dosing frequencies available, allowing for flexible adjustments in clinical trials to explore the optimal dosing dose and frequency based on patient-specific circumstances. ESG401 demonstrates clear efficacy in treating locally advanced/metastatic breast cancer after multiple lines of treatment. ESG401 shows good efficacy in patients with visceral metastases and clear therapeutic effects on intracranial lesions. These findings suggest that ESG401 may become an effective treatment option for breast cancer, especially visceral metastases, and as a Trop2-targeted ADC therapy, ESG401 shows potential therapeutic advantages.
About Escugen Biotechnology
Founded in 2017 by veteran returnees with decades of experience in antibody drug discovery, process development, and quality management in both international and domestic biopharmaceutical companies, Escugen Biotechnology focuses on the development of innovative ADCs. By leveraging target innovation, discovering differentiated competitive advantages, and expanding differentiated clinical pathways through specific combination therapies, Escugen continuously introduces next-generation innovative drugs. Escugen also has a team covering the entire professional chain of biopharmaceutical R&D, with functions spanning antibody engineering, new drug development, bioconjugation, preclinical research, process and quality research, and especially the scarce clinical R&D in the industry.
In addition to ESG401, Escugen has established its next-generation ADC technology platform with independent intellectual property rights, using efficient and low-toxicity camptothecin-based payloads. Compared to existing GGFG-Dxd class ADCs, this platform not only has higher antitumor activity but also strong resistance to drug resistance. It also demonstrates strong and durable antitumor activity in low-abundance, high-heterogeneity target tumors and high-load models, with excellent pharmacokinetic characteristics. Escugen is leveraging this opportunity to actively layout a series of innovative targets and ADC pipelines with greater safety and efficacy advantages through diverse collaborations.