On August 30, 2024, the results of the first-in-human Phase Ia dose-escalation study of Escugen's core product ESG401 were officially published online in the renowned international medical journal Cell Reports Medicine (impact factor: 11.7), a subjournal of Cell. The study was jointly led by the Cancer Hospital of the Chinese Academy of Medical Sciences and the Second Affiliated Hospital of Zhejiang University School of Medicine. Professors Ma Fei and Qiu Fuming served as the co-corresponding authors, while Dr. Wang Jian and Professor Tong Zhongsheng were the co-first authors. The publication of these results in Cell Reports Medicine highlights the international academic community's high recognition of the innovative development and clinical research outcomes of ESG401.

Research Background
ESG401 is a novel antibody-drug conjugate (ADC) that links the topoisomerase I inhibitor SN-38 to a humanized Trop2 IgG1 monoclonal antibody via a cleavable and stable linker, with a drug-to-antibody ratio (DAR) of 8. In terms of pharmaceutical properties, ESG401 exhibits high stability and controllability. Preclinical studies have shown that ESG401 has excellent safety, efficacy, and pharmacological characteristics. These results support the further clinical development of ESG401. The ESG401-101 study is a meticulously designed Phase I clinical trial aimed at evaluating the safety, tolerability, pharmacokinetic profile, and preliminary efficacy of ESG401 in patients with metastatic advanced solid tumors, further validating its structural innovation and preclinical study results.
Research Methods
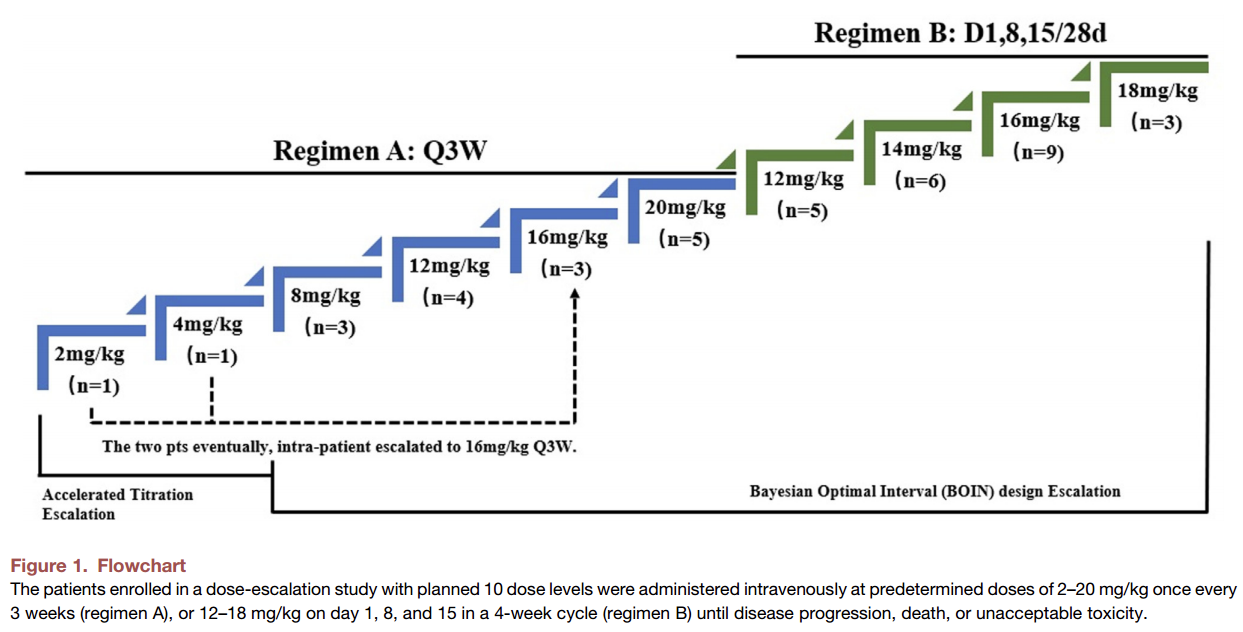
The Phase Ia dose-escalation study of the ESG401-101 trial (NCT04892342) included 10 dose levels and 2 dosing frequencies (see Figure 1). The study enrolled patients with advanced or metastatic solid tumors who had no effective standard treatment or were intolerant to standard treatment. The primary endpoint was the safety and tolerability of ESG401 in these patients, while secondary endpoints included objective response rate (ORR), disease control rate (DCR), duration of response (DOR), and progression-free survival (PFS), all assessed according to RECIST v1.1 criteria.
Research Results
A total of 40 patients with advanced or metastatic solid tumors were enrolled in the study, including 18 patients with hormone receptor-positive/HER2-negative breast cancer, 18 patients with triple-negative breast cancer, and 1 patient each with endometrial cancer and adenoid cystic carcinoma. All patients were female, with a median age of 53 years. The median number of previous treatment lines in the metastatic setting was 4 (range 1-12). All patients had distant metastases at baseline, with 10% having brain metastases, 63% having liver metastases, and 60% having lung metastases.
01 Safety
As of the data cutoff date (April 12, 2024), all 40 patients who had received at least one dose of ESG401 and had at least one post-dosing safety assessment were included in the safety analysis. A total of 38 patients experienced treatment-emergent adverse events (TEAEs), with an overall incidence rate of 95.0% (38/40). The most common TEAEs related to the study drug and occurring at a rate of ≥20% are listed in the table below. The most frequent ≥Grade 3 adverse events related to the study drug included neutropenia (40.0%), leukopenia (37.5%), and anemia (10.0%). No ≥Grade 3 diarrhea was reported.
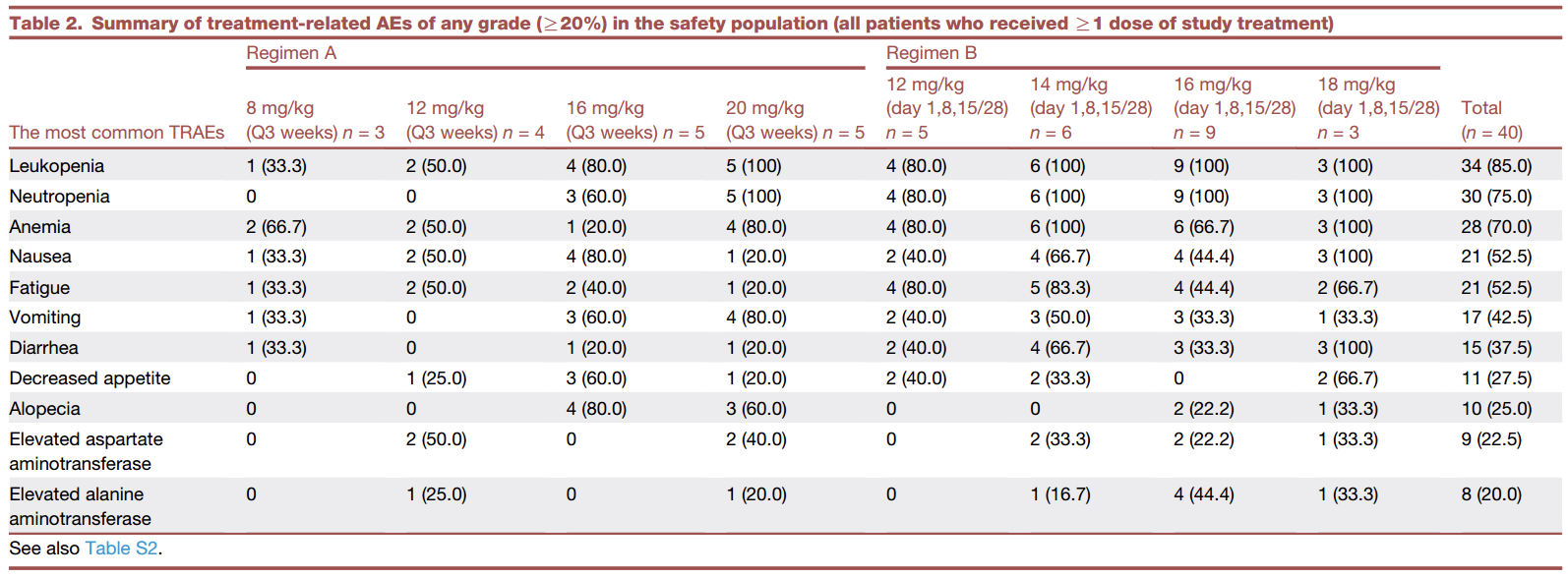
During the dose-escalation study, three patients experienced dose-limiting toxicities (DLTs) as defined by the protocol: one patient with Grade 4 neutropenia lasting >5 days and Grade 3 febrile neutropenia (20 mg Q3W dose group), one patient with Grade 3 febrile neutropenia (18 mg/kg D1,8,15/28d dose group), and one patient with Grade 4 neutropenia lasting >5 days (18 mg/kg D1,8,15/28d dose group). The maximum tolerated dose (MTD) was determined to be 16 mg/kg D1,8,15/28d.
02 Efficacy
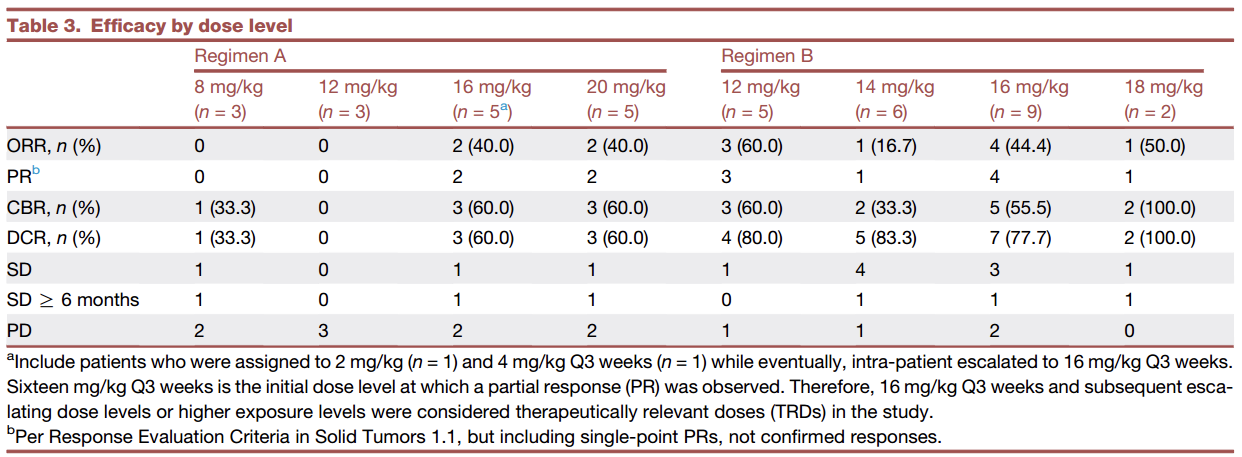
Among the 38 evaluable patients, the ORR was 34.2% (95% CI: 19.6, 51.4), and the DCR was 65.8% (95% CI: 48.6, 80.4). The efficacy results for each dose group are shown in the table below. In patients with HR+/HER2- breast cancer who received the treatment-related dose (TRD) and were evaluable for efficacy, the ORR was 47.0% (95% CI: 23.0, 72.2), and the DCR was 70.6% (95% CI: 44.0, 89.7). In patients with advanced triple-negative breast cancer, the ORR was 29.4% (95% CI: 10.3, 56.0), and the DCR was 52.9% (95% CI: 27.8, 77.0).
Conclusions
ESG401 demonstrated favorable safety, tolerability, and promising preliminary antitumor activity in patients with advanced solid tumors. These results support the further investigation of ESG401 in larger Phase II/III studies and the exploration of its efficacy in combination with immunotherapy or other targeted therapies.
Original article link in Cell Reports Medicine:
https://www.cell.com/cell-reports-medicine/fulltext/S2666-3791(24)00428-2
About Cell Reports Medicine
Cell Reports Medicine, a subjournal of Cell, was launched in 2020 and received its first impact factor in 2021. Published by the renowned international publisher ELSEVIER, it focuses on publishing cutting-edge research in translational and clinical biomedical sciences. The journal is issued 12 times a year, and its editor-in-chief is Professor Sara Hamilton from the United States. The journal is currently indexed in numerous databases, including SCIE. It has an ISSN of 2666-3791 and an eISSN of 2666-3791. It is ranked in Zone 1 by JCR and Zones 1/2 by the Chinese Academy of Sciences, with the latest impact factor of 11.7 and a five-year impact factor of 14.3.
About Escugen Biotechnology
Established in 2017 in the Zhangjiang Hi-Tech Park, Pudong New Area, Shanghai, Escugen Biotechnology focuses on the development of ADC new drugs. The company was founded by veteran returnees with decades of R&D experience in both international and domestic biopharmaceutical companies. The founding team has accumulated rich and successful R&D experience in leading biopharmaceutical companies both domestically and internationally, with capabilities covering antibody discovery, bioconjugation, process development and quality research, preclinical and clinical studies. Escugen's lead clinical pipeline, ESG401, is a Trop2-targeted ADC that uses an innovative stable and degradable linker to significantly reduce off-target toxicity. Clinical data suggest that ESG401 has a higher tolerable dose than other ADCs targeting the same antigen, with lower incidence and severity of off-target and on-target toxicities, demonstrating a clear safety advantage. The increased ADC dose and in-body exposure have led to encouraging efficacy in patients with advanced, heavily pretreated breast cancer, with significant antitumor effects on both visceral and intracranial metastases. Currently, ESG401 has initiated a pivotal Phase III clinical trial.