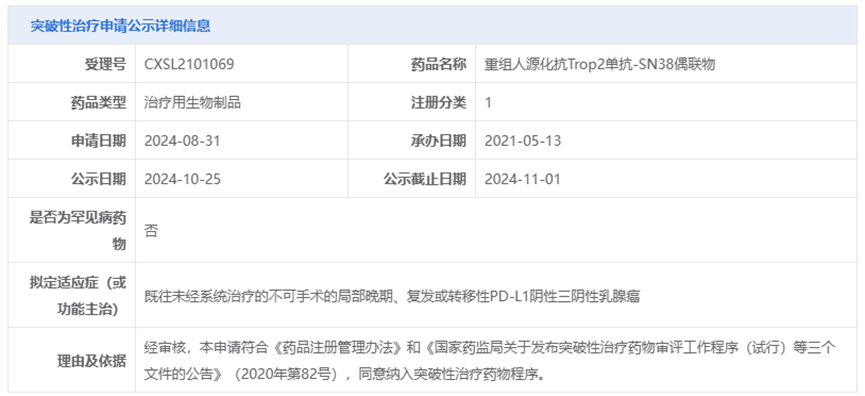
On November 2, 2024, according to the official website of the Center for Drug Evaluation (CDE) of the National Medical Products Administration (NMPA) of China, Escugen's core product ESG401 (recombinant humanized anti-Trop2 monoclonal antibody-SN38 conjugate) was granted Breakthrough Therapy Designation (BTD). The proposed indication is for the treatment of locally advanced, recurrent, or metastatic PD-L1 negative triple-negative breast cancer (TNBC) that has not previously been treated with systemic therapy.
ESG401 is Escugen's first ADC in the clinical stage of development. It is composed of the topoisomerase I inhibitor SN-38 linked to a humanized Trop2 IgG1 monoclonal antibody via a stable cleavable linker, with a drug-to-antibody ratio (DAR) of 8. Clinical data suggest that ESG401 has a higher tolerable dose than other ADCs targeting the same antigen, with lower incidence and severity of off-target and on-target toxicities, demonstrating a clear safety advantage.
The indication for which ESG401 was granted Breakthrough Therapy Designation (BTD) is "locally advanced, recurrent, or metastatic PD-L1 negative TNBC that has not previously been treated with systemic therapy."
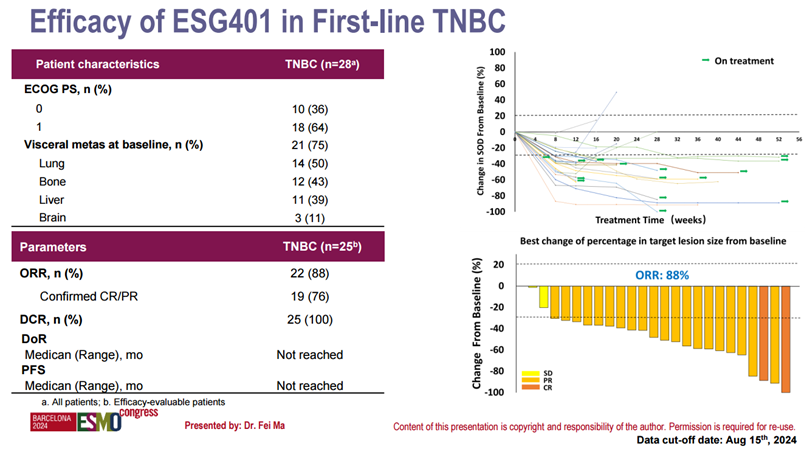
At the 2024 European Society for Medical Oncology (ESMO) Annual Meeting held in September, Professor Ma Fei from the Chinese Academy of Medical Sciences presented two mini oral reports on the clinical research results of ESG401, one of which included interim results of ESG401 in the cohort of patients with metastatic TNBC (mTNBC) treated in the first line: as of August 15, 2024, among the 25 evaluable patients in the ESG401 first-line mTNBC cohort, 2 patients achieved complete response (CR), and 20 patients achieved partial response (PR), with an objective response rate (ORR) of 88%; the disease control rate (DCR) was 100%. Notably, among the 3 patients with brain metastases in this cohort, 2 patients achieved intracranial complete response (IC-CR), and 1 patient achieved intracranial partial response (IC-PR), with an intracranial disease control rate (IC-DCR) of 100%.
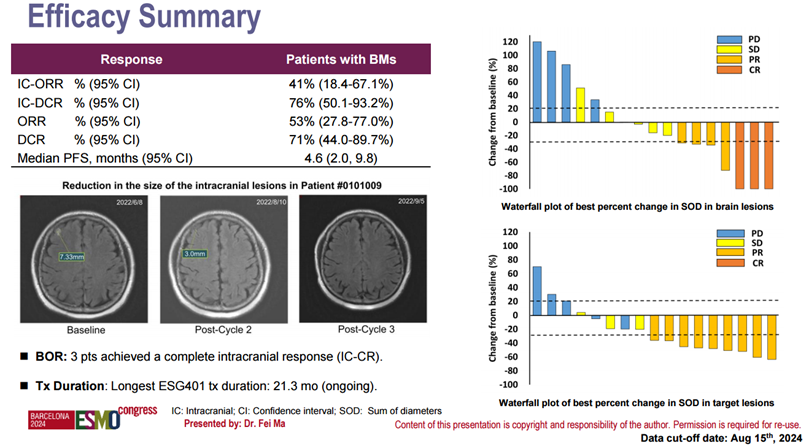
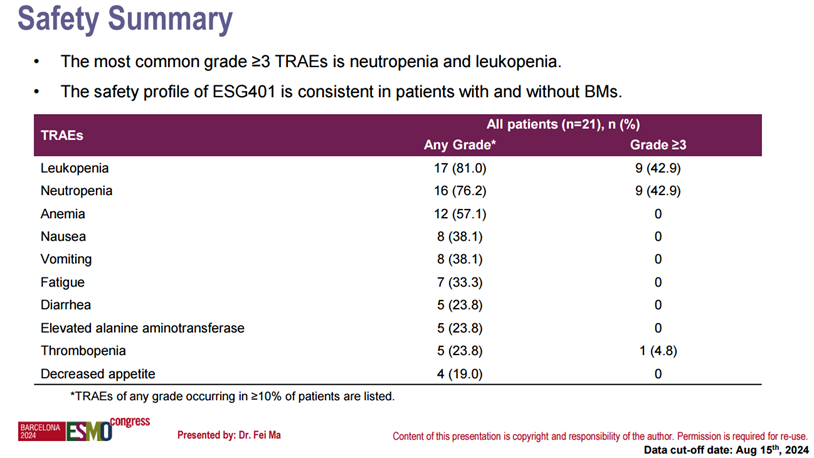
In another mini oral report at the 2024 ESMO Annual Meeting, data on the safety and efficacy of ESG401 in HER2-negative breast cancer patients with brain metastases at baseline were presented. This analysis included 21 patients with advanced breast cancer and brain metastases at baseline, of whom 67% had TNBC and 33% had HR+/HER2- breast cancer. The largest brain lesion measured 21 mm. Preliminary results showed that among the 17 evaluable patients after ESG401 treatment, 3 patients achieved intracranial complete response (CR), and 4 patients achieved partial response (PR), with an intracranial objective response rate (IC-ORR) of 41% and an intracranial disease control rate (IC-DCR) of 76%. The overall therapeutic response of these patients was consistent with the intracranial response, with an overall objective response rate (ORR) of 53% and an overall disease control rate (DCR) of 71%. Additionally, the safety profile of ESG401 in patients with brain metastases was consistent with that of the overall population.
Previously, ESG401 had received regulatory approval for the indication of "HR+/HER2- metastatic breast cancer previously treated with at least one line of systemic chemotherapy" and entered the pivotal Phase III clinical trial stage, with the first patient enrolled in July 2024.
About Escugen Biotechnology
Escugen is a clinical-stage biotechnology company located in Shanghai, China, focusing on the development of innovative ADC (antibody-drug conjugate) drugs. Currently, Escugen's lead Trop2 ADC pipeline, ESG401, has entered Phase III clinical trials. Escugen's next-generation linker-payload technology platform, EZWi-Fit®, offers significant competitive advantages in terms of safety, efficacy, anti-multiple drug resistance, and pharmacokinetic characteristics. Leveraging this platform technology, Escugen is rapidly expanding its ADC pipeline targeting new or validated targets. Escugen has also successfully licensed this platform technology to several domestic and international biotechnology companies to empower their innovative ADC projects.