On December 7, 2025, at the 67th ASH® Annual Meeting, Escugen presented via poster the first clinical data from its Phase 1/2 trial of ESG206 for patients with relapsed/refractory primary immune thrombocytopenia. ESG206 is a glycoengineered humanized anti-BAFF-R monoclonal antibody.
Study Title: Interim Report of a Phase 1/2 Study of ESG206 in Patients with Refractory or Relapsed Primary Immune Thrombocytopenia.
Introduction
Blockade of the BAFF-R signaling pathway and depletion of BAFF-R–expressing B cells may offer clinical benefit to patients with immune thrombocytopenia (ITP). ESG206, a glycoengineered humanized anti–BAFF-R monoclonal antibody, efficiently blocks B-cell activation, proliferation, and survival, and at the same time enhances ADCC towards B-cell depletion. ESG206 has shown promising efficacy and good tolerability in relapsed/refractory (R/R) B-cell malignancies and is being investigated for ITP. This report presents preliminary safety, PK, PD, and efficacy data from the Phase 1 part of an ongoing study (NCT06853444) of ESG206 in patients with primary ITP.
Methods
This Phase 1, open-label, multicenter, dose-escalation study enrolled patients with primary ITP who were previously treated with at least a corticosteroid (CS), and had a platelet count < 30 × 109/L. Patients received ESG206 at 1, 3, 6, or 9 mg/kg intravenously every 4 weeks for up to 4 doses. Concomitant CS and/or TPO-RA were allowed if the dosage was stable at least 14 days before the first ESG206 infusion. The primary objective is safety and tolerability. Secondary endpoints include PK, PD, and efficacy outcomes, such as (complete) response rates and confirmed (complete) response rates over time, time to response, and sustained response rates. Patients will be followed until 24 weeks after the first infusion to assess response durability.
Results
Up to 1 Aug 2025, 10 patients received at least one dose of ESG206 (Median [range] age, 40 [25–63] years; 3 males [30%]). Two patients received 3 infusions, 4 received 2, and 4 received 1 (two of them discontinued early due to personal decision). Median (range) time from ITP diagnosis was 57.4 (1.8–317.6) months, and median (range) baseline platelet count was 11.5 (0–19) × 109/L. Patients were previously treated with a median (range) of 4 (1–7) prior ITP therapies, including CS (100%), TPO-RAs (40%), rituximab (20%), and other immunosuppressants. ESG206 was given as monotherapy in 7 patients; while the other 2 received CS and 1 received TPO-RA as background therapy.
During treatment, 5 patients (50%) experienced adverse events (AEs), and most of them were Grade 1-2 and transient. Treatment-related AEs included rash (n=1, Grade 3) and fever (n=1, Grade 1). No patient discontinued treatment due to AEs. Pharmacokinetic results indicated a dose‑dependent increase in ESG206 plasma concentrations, with a half‑life of approximately 7 days across the 1–3 mg/kg dose range. PD assessment showed significant reductions of CD20+ B cells from baseline. In the 1 mg/kg group, all 4 patients had ≥ 90% reduction at Week 5; 3 of them achieved ≥ 98% reduction by Week 9. In the 3 mg/kg group, 3 patients showed ≥ 99% reduction at Week 5.
By cutoff, 5 patients (50%) achieved Response (platelet count ≥50 × 109/L without rescue therapy for at least 4 weeks prior to assessment and no additional ITP treatment before confirmed response). Three patients (30%) achieved Confirmed Response (≥50 × 109/L at more than 2 assessments at least 7 days apart). Five patients (50%) achieved Complete Response (platelet count ≥ 100 × 109/L without rescue therapy). Among responders, median (range) time to response was 7 (7–21) days.
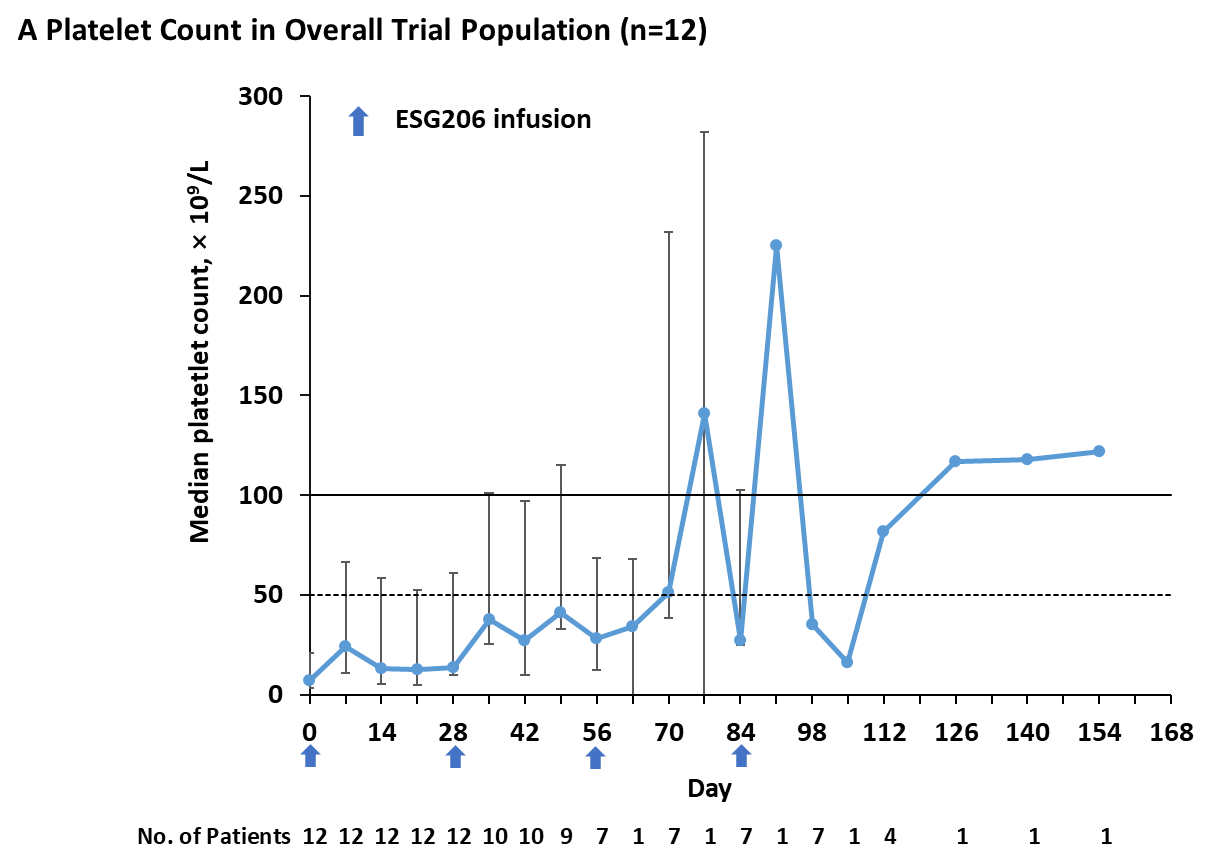
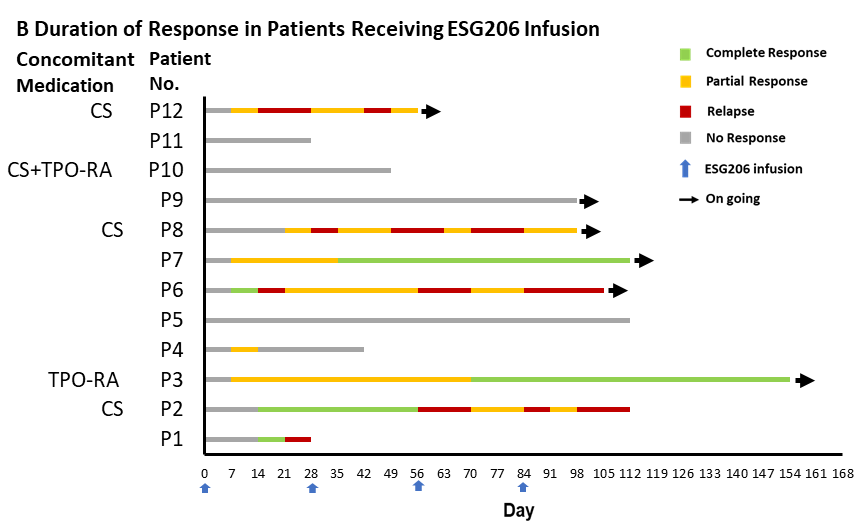
Figure 1. Platelet Count Dynamics and Duration of Response after ESG206 Infusion. Panel A shows the median platelet counts among all 12 enrolled patients. In Panel A, bars indicate the interquartile range, the solid horizontal line represents a platelet count of 100 × 109/L, and dashed lines represent platelet counts of 50 × 109/L. Panel B represents the swimmer’s plot of duration of response in individual. A complete response was defined as a platelet count of at least 100 × 109/L and the absence of bleeding. A partial response was defined as a platelet count of at least 30 × 109/L with an increase of at least twice the baseline count and the absence of bleeding. No response was defined as a platelet count below 30 × 109/L, or less than twice the baseline count, or the presence of bleeding. A relapse was defined as any of the following conditions occurring after a response: a platelet count below 30 × 109/L, or less than twice the baseline count, or the presence of bleeding. Concomitant use of thrombopoietin-receptor agonists (TPO-RAs) or glucocorticoids (CS) is indicated.
Key Highlights
These represent the first data on ESG206 in patients with primary ITP. Preliminary findings suggest that partial administration of the ESG206 treatment schedule already demonstrated rapid and encouraging efficacy with a favorable tolerability profile in heavily pretreated patients with relapsed/refractory ITP. These early interim results support further clinical exploration of ESG206 in this population. Meanwhile, clinical studies of ESG206 for other autoimmune diseases are ongoing, with Phase II trials for primary Sjögren's syndrome being initiated and clinical trials for systemic lupus erythematosus and other indications under planning.
The original poster of ESG206 presented at the ASH Congress is as follows:
